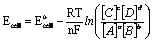L1 /
7
CH3041

pE AND pE0

Ox + ne- ® Red Eo1/2
Using activities: K1/2 = {Red} / ({Ox} {e-}n)
Using activities: K1/2 = {Red} / ({Ox} {e-}n)
Þ {e-}n =
{Red}/({Ox} K1/2) Using Laws of Logs:
pE =1/n log K1/2 -1/n
log {Red}/{Ox} = -log{e-}
pE = pEo - 1/n log {Red}
/ {Ox}
• pEo = 1/n log K1/2 = Eo1/2 / 0.0591
• The Nernst
Equation for a half reaction in
the pE form is : (concs. for convenience)
the pE form is : (concs. for convenience)
pE = pEo - 1/n log
([Red] / [Ox]) at 25oC