L1 /
5
CH3041

ION ACTIVITY

• Ions in a
concentrated solution interact electrostatically and reduce the effective concentration available for chemical rxn.
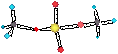
- recall MgSO4 ion pairing.
- recall MgSO4 ion pairing.
• To predict the
result of a rxn in an ionic solution
we need to account for the reduction
in effective concentration by
using an activity coefficient g .
using an activity coefficient g .
activity = concentration x g ai = [i(aq)] x gi
• The activity of an ion ai is the active concentation available to react (mol dm-3).
• A water with a
high acidity is said to have
a high proton activity. pH = - log { aH+ }
a high proton activity. pH = - log { aH+ }