L1 /
4
CH3041

EH

EH = Ecell in aqueous soln.
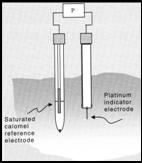
• To measure EH place a
platinum wire electrode in the
water to be tested + a reference
electrode (eg. calomel electrode).
platinum wire electrode in the
water to be tested + a reference
electrode (eg. calomel electrode).
The redox potential Ecell when
referenced back to the SHE
electrode it is called the EH value.
referenced back to the SHE
electrode it is called the EH value.
• EH values will be more positive in
an
oxidising environment. (eg. O2 EH +0.80V)
oxidising environment. (eg. O2 EH +0.80V)
• EH values will be more negative in
a
reducing environment. (eg. SO42- EH -0.27V)
reducing environment. (eg. SO42- EH -0.27V)
• ORP probes (Oxidation-Reduction-Potential)
measure using Pt relative to Ag/AgCl.
The results can be converted easily to EH.
measure using Pt relative to Ag/AgCl.
The results can be converted easily to EH.

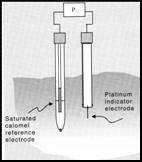
ORP probe
also
reads
out
in EH
also
reads
out
in EH
Values
measured in acid mine drainage waters
are quite accurate - not so for natural waters!
are quite accurate - not so for natural waters!