L1 /
14
CH3041

HIGH CHARGE METAL
IONS
• Small ions with high
charge (+6, +4, +3) react
with water:
with water:
• some
(particularly the transition metal ions)
are made less soluble:
Fe3+ + 3H2O ® Fe(OH)3(s) + 3H+
are made less soluble:
Fe3+ + 3H2O ® Fe(OH)3(s) + 3H+
Al3+ + 3H2O ® Al(OH)3(s) + 3H+
(Gibbsite)
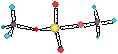
• some non-metal cations are made more
soluble:
S6+ + 4H2O ® SO42- + 2H+
soluble:
S6+ + 4H2O ® SO42- + 2H+