L1 /
11
CH3041

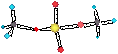
WATER HARDNESS

• Ca2+ & Mg2+ are the cations usually found
with CO32- / HCO3- for charge balance.
with CO32- / HCO3- for charge balance.
• Water
hardness is a result of the presence of Ca2+, Mg2+ and sometimes Fe2+.
•Typically : Hardness = [Ca2+] +
[Mg2+]
• Temporary hardness occurs for Ca(HCO3)2 and is lost on boiling the water where a
precipitate forms. Ca(HCO3)2(aq) ® CaCO3(s)Œ + CO2(g) + H2O(l)
precipitate forms. Ca(HCO3)2(aq) ® CaCO3(s)Œ + CO2(g) + H2O(l)
• Permanent hardness occurs with CaSO4.
• Hardness
measurements: complexiometric titrations with EDTA as mg CaCO3 eq. / L
mmol Ca2+ (by EDTA) x 100 (MW CaCO3)
mmol Ca2+ (by EDTA) x 100 (MW CaCO3)
• Soft 0 - 50, Hard
200 – 300 meq/L
Tablelands Chillagoe borewater
Tablelands Chillagoe borewater