L1 /
7
CH3041

MAJOR ACID
COMPONENTS

H2CO3 (carbonic
acid) (weak acid)
• Carbonate rocks are ubiquitous and, the
presence of CO2 as a minor atmospheric gas, means that this is the most important
acid / base system in the environment.
presence of CO2 as a minor atmospheric gas, means that this is the most important
acid / base system in the environment.
Humic
and Fulvic acids (weak acids)
• Polyprotic acids (can lose more than 1 H+)
Complex polymeric organic acids
Humic substances can be pptd at pH
= 2
Fulvic
substances stay
in soln at pH = 2
(have more COOH and -OH groups)
(have more COOH and -OH groups)
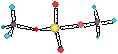
HCl, H2SO4,
HNO3 (strong acids)
• Volcanic
sources (also
called mineral acids)