L1 /
5
CH3041

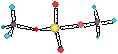
ALKALINITY III

The major basic
ions present in natural
waters are HCO3-, CO32-, OH- .
waters are HCO3-, CO32-, OH- .
Concentration
dependence
acidic pH H2CO3 = CO2
slightly acidic H2CO3, HCO3-
slightly
basic HCO3-, CO32-
basic
pH CO32-, OH-
The alkalinity serves as
a
reservoir for inorganic carbon and thus as an indicator of the ability of the water to support biota it is also a buffer system.
reservoir for inorganic carbon and thus as an indicator of the ability of the water to support biota it is also a buffer system.
As Dissolved Inorganic Carbon (pH7 HCO3-)
is used up a water will become more basic.
is used up a water will become more basic.
Eventually the
water becomes too basic &
algal biomass production stops.
algal biomass production stops.
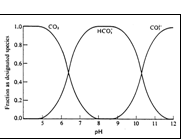
Species Distribution Diagram