L1 /
18
CH3041

OXIDANTS,
REACTIVE SUBSTANCES
REACTIVE SUBSTANCES

3) Oxidants
Oxidants react with combustible materials (reductants) to produce heat. A flammable & a strong oxidant may burn explosively.
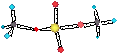
NH4VNO3(s) ® IN2O(g) + 2H2O(g)
-IVCH4 ® IVCO2
-IVCH4 ® IVCO2
Heating
this oxidant with an organic fuel will give an explosion! (Oklahoma State
bombing)
Some other
common oxidants are:
halogens, hydrogen peroxide, HNO3.
4) Reactive
substances
Substances which react violently - either spontaneously (NI3) or with water, acid, base or air. They may produce toxic fumes such as H2S &
HCN. eg.
Na + H2O ®
2NaOH + H2