L1 /
13
CH3041

ANTARCTIC OZONE
HOLE III

• A heterogeneously
catalysed reaction
occurs on the surface of PSC ice crystals.
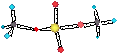
ClONO2(g)+ HCl(g)® Cl2(g)+ HNO3(aq)
This happens all through winter.
occurs on the surface of PSC ice crystals.
ClONO2(g)+ HCl(g)® Cl2(g)+ HNO3(aq)
This happens all through winter.
• Polar spring, sunlight returns & with it
O3
formation but: 1Cl2(g)+ hn ®2Cl.(g)
formation but: 1Cl2(g)+ hn ®2Cl.(g)
• The net result is rapid
generation of Cl.
followed by massive loss of O3.
followed by massive loss of O3.
• This is a
transient phenomenon by mid-
spring the ice crystals melt and release
NOx. The catalytic reaction stops, the NOx
traps OCl. and normal O3 levels return.
spring the ice crystals melt and release
NOx. The catalytic reaction stops, the NOx
traps OCl. and normal O3 levels return.