L1 /
3
CH3041

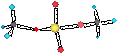
E = mc2

• Enucleus < S Eisolated-nucleons
• E = mc2 Ž mnucleus < S mnucleons
• Dm is the mass
defect for a nucleus.
Nucleus: Dm = mnucleus - S mnucleons
• DEBE = Dmc2 Einstein’s
equation
the relation between the change in nuclear binding energy (DEBE) & the mass defect.
the relation between the change in nuclear binding energy (DEBE) & the mass defect.
• For the
chemical reaction H2 + ½O2 ® H2O
1 mol of H2 releases DHof -286 kJ which is equivalent to » 3ng of mass.
1 mol of H2 releases DHof -286 kJ which is equivalent to » 3ng of mass.
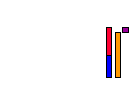
S EBE(Ba + Kr)
S EBEU
D EBE
• The fission of
uranium forms two nuclei
with more binding E per nucleon
(more stable), the DEBE is released as heat.
with more binding E per nucleon
(more stable), the DEBE is released as heat.
• 1 mol of 235U releases -2.1x1010 kJ