L1 /
4
CH3041

ION CHROMATOGRAPHY
II

• An ion exchange resin loaded with 1 type of
ion (eg. H+) will bind
small amounts of a different ion
almost quantitatively. (eg. Na+)
•Selectivity is higher for: higher charge ions,
smaller hydration radius, high polarizability.
(selectivity = strength of bonding to resin)
smaller hydration radius, high polarizability.
(selectivity = strength of bonding to resin)
eg. Ca2+ >Cu2+ >Zn2+ >K+ >NH4+ >Na+ >H+ >Li+
ie. Ca2+ binds more strongly than H+
ie. Ca2+ binds more strongly than H+
Ion
Chromatography
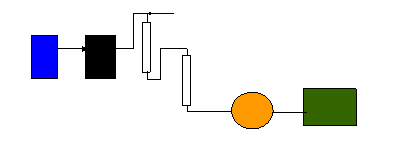
detector
integrator/PC
membrane
ion supressor
ion supressor
pump
Mobile
phase
reservoir
phase
reservoir
column
sample