L1 /
9
CH3041

ION SELECTIVE
ELECTRODES

pH
Electrodes
a) glass
electrode
(CH1011)
(CH1011)
b) semiconductor
pH sensor (CH3041)
pH sensor (CH3041)
• In both
electrodes an
H+ sensitive layer , (eg. a glass membrane) is
exposed to the solution containing [H+]unknown
H+ sensitive layer , (eg. a glass membrane) is
exposed to the solution containing [H+]unknown
• On the other side
of the layer an electrode
senses the change in potential as a result of
the new H+ concentration, this changes the
voltage relative to a reference electrode
( eg. external Ag/AgCl ) \ a new Ecell results
senses the change in potential as a result of
the new H+ concentration, this changes the
voltage relative to a reference electrode
( eg. external Ag/AgCl ) \ a new Ecell results
• display : pH = ( Ecell - EAg/AgCl + k) / 0.059
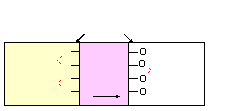
O
O
O
O
-
-
-
-
-
-
-
-
H+
H+
H+
H+
H+
H+
H+
H+
H+ H+
H+
Glass
Exchange sites
occupied by Na+ or H+
occupied by Na+ or H+
Internal External
high
pH
pH
Na+