L1 /
12
CH3041

ATOMIC SPECTROSCOPY
I

• A sample of
liquid containing the analyte is placed
into a flame (2500K acetylene + air) which
results in atomisation. In AES the atoms* emit light which, after l
selection by the monochromator,
is detected. In AAS a light
beam is fed into the flame the atoms absorb
light and then the difference is measured.
beam is fed into the flame the atoms absorb
light and then the difference is measured.
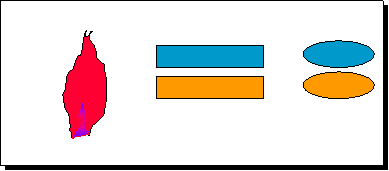
Flame
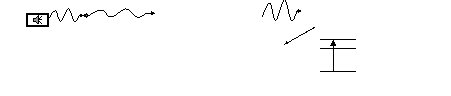
Light
Source
Source
(Fe hollow
cathode)
cathode)
Monochromator
Detector
Atomic absorption signal
(AAS)
(AAS)
Excited
States
Ground State
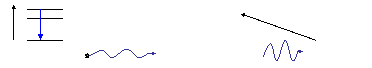
Monochromator
Detector
Atomic
emission signal (AES)
E
Atom