L1 /
1
CH3041

ON-LINE LECTURE 19

GRAVIMETRY relies on the use of an analytical balance.
• In most laboratories this will weigh to
the
sub-mg level. To do this reliably it must be
kept clean & level, used in a draft & vibration free area and be calibrated regularly (monthly) using a standard mass.
sub-mg level. To do this reliably it must be
kept clean & level, used in a draft & vibration free area and be calibrated regularly (monthly) using a standard mass.
• (1)
Gravimetric analysis: An analyte is reacted with a precipitant to bring about precipitn. The product(s) is weighed & the mass is
related back through the product formula to the no. of moles of
the analyte.
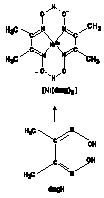
eg. Ni2+ + 2 DMG ® Ni(DMG)2(s) (CH1012)
eg. Ni2+ + 2 DMG ® Ni(DMG)2(s) (CH1012)