L1 /
13
CH3041

SILICATES II

• Extra cations
give charge neutrality.
• eg. orthoclase feldspar KAlSi3O8
Al occupies 1 in every 4 tetrahedral sites,
the -ve charge is neutralised by the K+
Al occupies 1 in every 4 tetrahedral sites,
the -ve charge is neutralised by the K+
•The
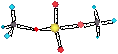
bonding in the SiO44- tetrahedra has significant covalent
character.
• When a mineral dissolves the Si-O linkages are
much stronger than the ionic cation-anion interactions and will
break last.
Polymeric Silicates
Connection of the SiO4 tetrahedra may occur through 1 - 4 of the oxygen atoms.
Connection of the SiO4 tetrahedra may occur through 1 - 4 of the oxygen atoms.