L1 /
12
CH3041

DISTRIBUTION
COEFFICIENT

• Freundlich Isotherm [is] = KF [iw] n
• KF & n are constants obtained by plotting the adsorption of
[is] as a function of [iw].
• log
[is] = n x log [iw] + log KF
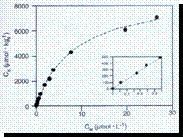
• The example shown
is for the adsorption:
1,4-dinitrobenzene on K+-illite, pH 7, T 25oC
1,4-dinitrobenzene on K+-illite, pH 7, T 25oC
• [is] = 1000 [iw] 0.7 ([iw] =15) Þ Kdist = 450 l kg-1
• Adsorption
of the herbicide 2,4-D on a
clay-fulvic acid complex obeys a similar eqn.
clay-fulvic acid complex obeys a similar eqn.
• Isotherms
allow prediction of surface
loadings to be made in advance over a
defined concentration range.
loadings to be made in advance over a
defined concentration range.
KF =
Freundlich constant
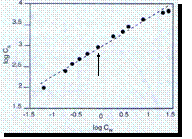
n = 2 / 2.85
KF = 10 3