L1 /
9
CH1012

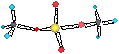
SILICIC ACID

Over 90%
of the Earths crust is silicate
material. The covalent silicate structures are very strong but they do break slowly under carbonic acid attack to generate
the hard to characterise silicon hydroxide or silicic acid. H4SiO4 = Si(OH)4
material. The covalent silicate structures are very strong but they do break slowly under carbonic acid attack to generate
the hard to characterise silicon hydroxide or silicic acid. H4SiO4 = Si(OH)4

[SiO44-](s) + 4H+ ® Si(OH)4(aq)
• Silicic acid is
involved in silicon
cycling in the oceans where
marine phytoplankton (diatoms)
incorporate amorphous silicon
oxides in their cell walls.
cycling in the oceans where
marine phytoplankton (diatoms)
incorporate amorphous silicon
oxides in their cell walls.