L1 /
3
CH1012

GROUP IVA
PROPERTIES

• Covalent bond character is of major importance in this group. Carbon forms the
most extensive range of compounds of any
element – organic + inorganic chemistry.
most extensive range of compounds of any
element – organic + inorganic chemistry.
• Elemental silicon and germanium are metalloid semiconductors; pure Si is used in making microprocessor chips, solar cells.
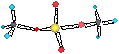
• Silicon forms very stable covalently bonded oxygen containing cmpds: silicates.
• Tin
and lead are much more metallic giving compounds
which are ionic in
character.