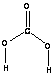L1 /
6
CH1012

CARBONIC ACID

Carbon dioxide is
present as a minor gas
in the atmosphere (0.038%). The reaction
of carbon dioxide with water forms
carbonic acid. CO2(aq) + H2O(aq) ® H2CO3(aq)
in the atmosphere (0.038%). The reaction
of carbon dioxide with water forms
carbonic acid. CO2(aq) + H2O(aq) ® H2CO3(aq)
• Carbonic acid H2CO3 is the most
important naturally occurring
acid. (this hydroxide is a weak
acid) H2CO3(aq) D HCO3-(aq) + H+(aq)
• The exogenic cycle treats
the crust of the Earth as a large
acid-base titration system.
Carbonic acid in rain water dissolves the basic rocks which are carried away to oceans. The components form sediments which are then compressed back into rocks.
Carbonic acid in rain water dissolves the basic rocks which are carried away to oceans. The components form sediments which are then compressed back into rocks.