L1 /
4
CH1012

CARBON CHEMISTRY

Carbon. occurs in nature in many
forms, >50% is as the carbonate
minerals eg calcite (CaCO3)
forms, >50% is as the carbonate
minerals eg calcite (CaCO3)
• May be found in
the elemental
forms graphite and diamond.
Which have network covalent
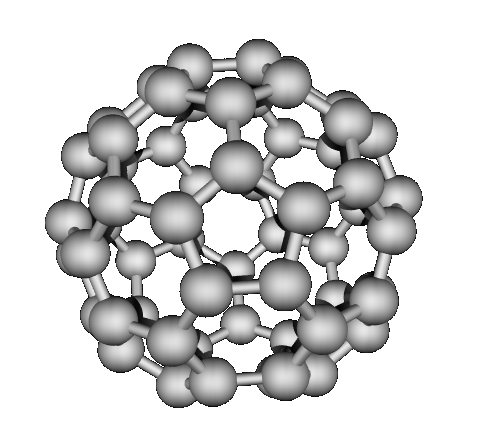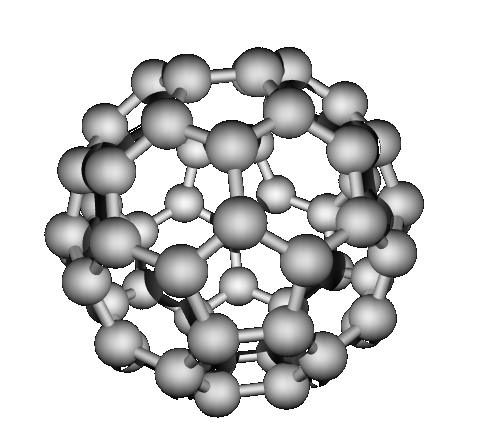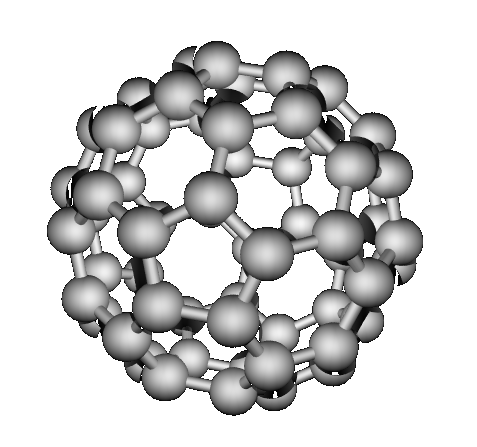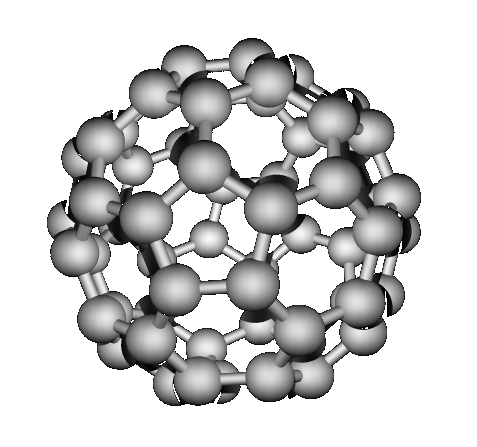
bonded structures. “Buckyballs” buckminsterfullerene (1985)
forms graphite and diamond.
Which have network covalent
bonded structures. “Buckyballs” buckminsterfullerene (1985)
• No tendency to form ionic
organic cmpds.
• Will bond with itself to form chains,
rings & branched structures and with
a wide variety of other elements :
H, O, N, S, halogens, transition metals.
rings & branched structures and with
a wide variety of other elements :
H, O, N, S, halogens, transition metals.