L1 /
10
CH1012

ACID EQUILIBRIA

Al metal dissolves in both acids & bases
2Al + 6H+ ® 2Al3+ + 3H2
2Al + 6H+ ® 2Al3+ + 3H2
2Al + 2OH- + 6H2O ® 2Al(OH)4- + 3H2
• Solutions of Al3+ in water
are acidic because of the equilibrium
are acidic because of the equilibrium

®
¬
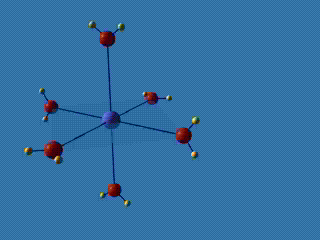
Al(OH2)63+ + H2O Al(OH2)5(OH)2+ + H3O+
• For the
"ionic metals",
solutions of the
the hydrated ions are
essentially neutral.
eg. Na+, Ca2+
solutions of the
the hydrated ions are
essentially neutral.
eg. Na+, Ca2+