L1 /
14
CH1012

METAL COMPLEXES

Metal complex formation
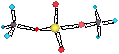
• Metal complexes are formed between a
metal cation and ligands - which are groups
which bond to the metal. The complex does not dissociate in solution, it exists as
a discrete molecular unit.
metal cation and ligands - which are groups
which bond to the metal. The complex does not dissociate in solution, it exists as
a discrete molecular unit.
• There are several known metal complexes of the group 1A and 2A metals a few examples of which will be mentioned.
Na+ + acac- ® (acac)Na(OH2)2 (in water)
( acac- = acetylacetonate = CH3COCHCOCH3- )