L1 /
12
CH1012

IONIC SOLIDS

•Ionic
solid: no discrete molecular units rather a 3-D lattice of cations(+), anions(-) in a
fixed arrangement. eg. Na Cl
•Strong electrostatic
attraction between each ion and neighbouring oppositely charged ions
holds the solid together.
This attraction is
termed ionic bonding.
holds the solid together.
This attraction is
termed ionic bonding.
•Ionic
bonding is common
between elements on the LHS & RHS of the periodic table, ie. metal + non-metal
between elements on the LHS & RHS of the periodic table, ie. metal + non-metal
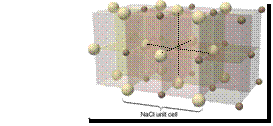
Cl-
Na+