CH1012

L1 /
‹#›
L1 /
13
SUMMARY: TRENDS IN ACIDITY

1) metal ®
metalloid ® non-metal
change is also reflected in changes in acidity as we go ACROSS a Period.
change is also reflected in changes in acidity as we go ACROSS a Period.
• LHS metal oxides/hydroxides: basic
eg. Li2O, LiOH, Na2O2, NaOH
eg. Li2O, LiOH, Na2O2, NaOH
• In the middle we have the amphoteric
oxides/hydroxides. eg. BeO, Be(OH)2, Al2O3
oxides/hydroxides. eg. BeO, Be(OH)2, Al2O3
• metalloid oxides/hydroxides: weakly
acidic
eg. B2O3, B(OH)3, Si(OH)4
eg. B2O3, B(OH)3, Si(OH)4
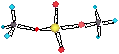
• RHS nonmetal oxides/hydroxides: acidic.
eg. H2CO3 = CO(OH)2,
(NO2, HNO3, P2O5, H2SO4)
eg. H2CO3 = CO(OH)2,
(NO2, HNO3, P2O5, H2SO4)