CH1012

L1 /
‹#›
L1 /
12
SUMMARY: IONIC RADII

• Cations are smaller than the corresponding atoms,
as the
valence shell of the atom is
vacated as the ion is formed.
valence shell of the atom is
vacated as the ion is formed.
• Group IIA cations are
smaller than the
corresponding Group IA
element as the eff. nuclear
charge will be greater for
the Group IIA ion
smaller than the
corresponding Group IA
element as the eff. nuclear
charge will be greater for
the Group IIA ion
eg. Na+ Z=11 / 10e,
Mg2+ Z=12 / 10e.
Mg2+ Z=12 / 10e.
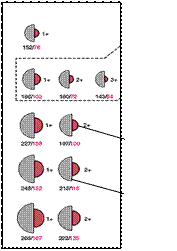
Li
Li
Na
Mg Al
K
Ca
Rb
Sr
Cs
Ba
1A 2A
3A
GROUP
GROUP
2
3
4
5
6
3
4
5
6
CATIONS
ATOMS