CH1012

L1 /
‹#›
L1 /
9
1A + O2

(b)
Reaction with oxygen
Group 1A + O2
Ø lithium ® Li2O (oxide; O2-)
Ø sodium ® Na2O2 (peroxide; O22-)
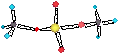
Ø K, Rb, Cs ® MO2 (superoxide; O2-)
• the differences are due to the different sizes of the
anions and cations and the most stable ionic lattice that results.
eg. small cation + small anion ® stable solid
eg. small cation + small anion ® stable solid
• Airpacks (firefighters) use O2 stored in KO2.