CH1012

L1 /
‹#›
L1 /
16
SPARES II

2) What is the main
difference between a
lyophillic colloid and a micelle?
lyophillic colloid and a micelle?
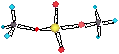
A lyophillic
colloid is a “solvent liking” 1 phase system – the colloid is soluble in
the
dispersion medium. If the dispersion medium is water the colloid will be polar
eg. protein+H2O
dispersion medium. If the dispersion medium is water the colloid will be polar
eg. protein+H2O
A micelle is an organised
aggregate of highly surface active materials (surfactants).
• The micelle is insoluble in the disp. medium. The
surfactants will either be polar or ionic which determines the size of the micelle. eg. sodium stearate (<100) + H2O