CH1012

L1 /
‹#›
L1 /
4
SN1 REACTIVITY

The reaction rate
is sensitive to electronic
factors which govern the stability of
the carbocation intermediate.
RCH2-X <<
R2CH-X < R3CX
1o 2o 3o

increasing
rate
increasing stability of C+
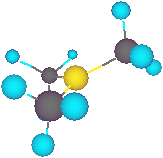
The formation of the ionic intermediate is
favoured with polar solvents & we are
therefore able to control the reaction so that
either the SN2 or SN1 mechanism will occur.
favoured with polar solvents & we are
therefore able to control the reaction so that
either the SN2 or SN1 mechanism will occur.
+