CH1012

L1 /
‹#›
L1 /
8
SN2 MECHANISM

1o alkyl halides RCH2-X always SN2
2o alkyl halides
R(R’)CH-X generally SN2
Experimentally
Rate (of
subs.) = k [RCH2X] [Nu:-]
Proposed Mechanism
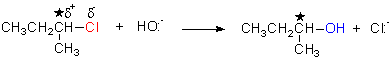
chiral
centre

S
R
nucleophile
leaving
group
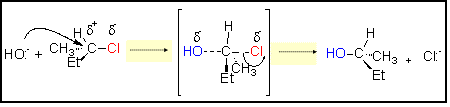
TS
plane
(Et = C2H5)