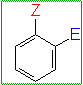
CH1012

L1 /
‹#›
L1 /
11
THE 2ND SUBSTITUTION

ortho-para directing
groups:
where Z = an electron donating grp.
where Z = an electron donating grp.
eg. -OH, -OR, -NR2, -Ph, -R, -F
meta directing
groups:
where Z = an electron withdrawing group.
where Z = an electron withdrawing group.
eg. -NO2,
-CN, -C(=O)R,
-C(=O)OR, -CF3
activating groups:
more reactive than C6H6
ortho-para directing & there are lone
pair(s) on the atom adjacent to the ring.
ortho-para directing & there are lone
pair(s) on the atom adjacent to the ring.
deactivating groups: less reactive
than C6H6
all the groups that are not covered within
the activating group classification.
all the groups that are not covered within
the activating group classification.