CH1012

L1 /
‹#›
L1 /
17
WORKING II
distance
Y
1
2
3

2) Use the
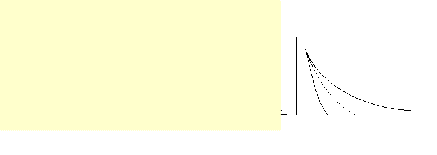
Gouy-Chapman model of the
diffuse double layer to predict why the
addition of 1.0 M BaCl2 to a colloid is
more successful than the addition of a
1.0 M solution of NaCl in causing the
colloid to flocculate.
diffuse double layer to predict why the
addition of 1.0 M BaCl2 to a colloid is
more successful than the addition of a
1.0 M solution of NaCl in causing the
colloid to flocculate.
The addition of the higher charge Ba2+ will result in a larger
reduction of the electric potential at the negative
surface of the colloid.
This reduces the
electrostatic repulsion
between particles and the
colloid flocculates.
surface of the colloid.
This reduces the
electrostatic repulsion
between particles and the
colloid flocculates.