CH1012

L1 /
‹#›
L1 /
12

- all Cs are identical
- all Hs are identical
- all C-C bond lengths 1.39 Å
- all angles 120o (Å = 10-10m)
• From this we take the Cs to
be in sp2 hybridization.
be in sp2 hybridization.
• The molecule is a cyclic, planar,
delocalised 6 p electron system.
• Aromaticity refers to the special stability of
(4n + 2 p) electron planar, cyclic systems
and benzene was the first example known.
(4n + 2 p) electron planar, cyclic systems
and benzene was the first example known.
AROMATIC COMPOUNDS
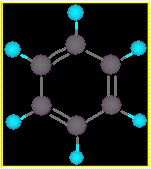
The structure of C6H6 :