CH1012

L1 /
‹#›
L1 /
14
ALKYNES

• Alkynes generally display very similar reactivity to
alkenes with bromination, hydration & hydrohalogenation
occurring.
A notable exception: terminal RCCH alkynes
• The H of terminal alkynes is weakly acidic pKa » 25 compared with alkenes pKa » 44.
• Treatment with a strong base gives an acetylide anion a very useful
nucleophile in organic synthesis.
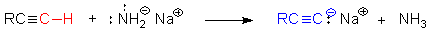
• The strong base here is sodium amide &
when R = H the product is sodium acetylide.
S.I. sodium ethynide
when R = H the product is sodium acetylide.
S.I. sodium ethynide