CH1012

L1 /
‹#›
L1 /
10
UV SPECTROSCOPY I

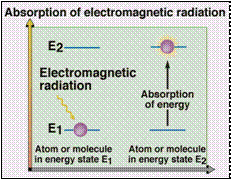
Absorption of UV irradiation (l 200 - 400
nm)
results in an electronic excitation.
results in an electronic excitation.
An electron is promoted from a low E
orbital
to a high E empty
orbital. Ground state
to an excited state.
to a high E empty
orbital. Ground state
to an excited state.
D E = hn
= hc / l
h = Planck’s constant
c = speed of light
n = frequency
l = wavelength
UltraViolet Spectroscopy