CH1012

L1 /
‹#›
L1 /
16
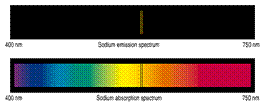
ABSORPTION / EMISSION

• The 2 most common types of spectra are:
absorption spectra where a light
beam is shone on the sample and
absorption spectra where a light
beam is shone on the sample and
• emission spectra where the
sample is placed in an excited
state which then decays back to
the ground state emitting
radiation. Ephoton = h c / l
sample is placed in an excited
state which then decays back to
the ground state emitting
radiation. Ephoton = h c / l
• Ephoton = Emolecule-highEstate - Emolecule-lowEstate