CH1012


L1 /
14
EQUILIBRIUM

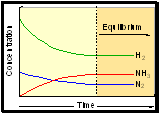
At Equilibrium:
• the amounts of reactants /
products do not change with time.
products do not change with time.
• rate of forward &
back reaction are equal
Kc = [product C]c
[reactant A]a [reactant B]b
[reactant A]a [reactant B]b
• a, b, c are from the
balanced equation
• Kc is the equilibrium constant which tells
us the extent of reaction at completion.
us the extent of reaction at completion.
Chemical
Equilibrium and Kc

k Kc

®
¬
aA + bB cC
kf
kr

Kinetics