CH1012

L1 /
‹#›
L1 /
8
AUFBAU / HUNDS RULE

Aufbau principle
For a ground state atom each electron enters the lowest energy orbital available.
For a ground state atom each electron enters the lowest energy orbital available.
eg. C: 1s2 2s22p2 not
1s22p4
Hunds Rule
When electrons are placed in a set of orbitals of the same energy (e.g. 3 x 2p orbitals) the electronic configuration with the lowest energy has the maximum number of unpaired e- with parallel spins.
When electrons are placed in a set of orbitals of the same energy (e.g. 3 x 2p orbitals) the electronic configuration with the lowest energy has the maximum number of unpaired e- with parallel spins.
eg. O 1s22s22p4

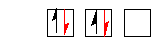
not
Pauli exclusion principle:
No 2 e- in the same atom can have the same
4 quantum no’s. n(1s), l(sÞ l=0), ml, ms(½,-½)
No 2 e- in the same atom can have the same
4 quantum no’s. n(1s), l(sÞ l=0), ml, ms(½,-½)
l
s = 0
p = 1
s = 0
p = 1