CH1012

L1 /
‹#›
L1 /
9
OTHER TRANSITIONS

¬
®
b) Solid Vapour
Sublimation

ln p = -DHsub/RT +
constant
Multiple stable phases may exist
for the same physical state eg. Ice I, Ice II ...
for the same physical state eg. Ice I, Ice II ...

¬
®
c) Solid Liquid
Melting
point varies slightly with pressure
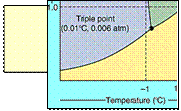
eg.
water mp. = 0.0000oC at 760 torr
= 0.0098oC at 4.58 torr
-
solids & liquids are nearly incompressable
P
CO2

1 atm
pressure
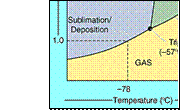
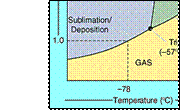
1) CO2(s) sublimes at -78oC
1) CO2(s) sublimes at -78oC
P
CO2
P
H2O

P
H2O
[ Ice melts at 0oC ]