CH1012

L1 /
‹#›
L1 /
17
HENRY’S LAW

3.
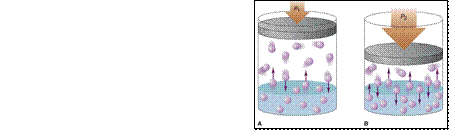
Two component systems
- gas / liquid mixtures
- gas / liquid mixtures
®
¬
gas +
solvent solution
Henry’s Law
[i ] = KH pi
[i ] - solubility of gas i (mol gas / dm3 of liquid)
KH - Henry’s law
constant (mol
/ dm3.atm
)
pi - press. of gas i in equil. with
liquid (atm.)
(N.B. This defn. is different from Zumdahl)
(N.B. This defn. is different from Zumdahl)