CH1012

L1 /
‹#›
L1 /
14
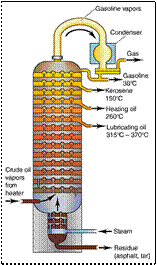
FRACTIONAL DISTILLATION

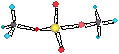
ptotal = pA + pB = poA xA + poB xB
• with ideal behaviour the
presence of the two
liquids lowers the V.P.
of each component
presence of the two
liquids lowers the V.P.
of each component
• the vapour has a higher
mole fraction of the more
volatile solution component.
mole fraction of the more
volatile solution component.
benzene(95torr):toluene(28torr)
liquid 50:50 vapour 77:23
eg. petroleum refining