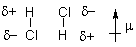CH1012

L1 /
‹#›
L1 /
4
VAN DER WAALS FORCES

Van der Waals forces
- intermolecular attractive forces
- intermolecular attractive forces
1) Dipole-dipole forces polar molecules
e.g. the b.p. of organic cmpds increases with increasing m
dipole moment
e.g. the b.p. of organic cmpds increases with increasing m
dipole moment
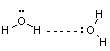
2) Hydrogen bonds polar molecules
typically involves either H-F, H-N or O-H
typically involves either H-F, H-N or O-H
eg. water
dissolves so many
substances because of it’s
high H-bonding ability
substances because of it’s
high H-bonding ability