L1 /
10
CH1012

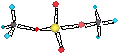
4-COORDINATE COMPLEXES I

Four-coordinate
metal complexes can have either tetrahedral or square-planar
stereochemistries and, in
fact, both types are quite
common.
• On purely electrostatic grounds, one would expect that 4-coordinate complexes would always assume the more symmetrical tetrahedral stereochemistry as this enables the ligands (bond pairs) to be separated by the maximum distance.
• eg. the d10-metal
ions adopt this geometry