L1 /
10
CH1012

COORDINATION CMPDS

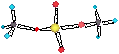
Werner formuled the complexes as [Co(NH3)6]Cl3 , [Co(NH3)5Cl]Cl2 and [Co(NH3)4Cl2]Cl where the complex
cations, (represented by the species inside the square brackets)
are discrete entities
in solution, each having
its own unique properties.
in solution, each having
its own unique properties.
Each Co centre
has six groups
strongly bound to it called ligands
(Latin "ligare", to bind)
strongly bound to it called ligands
(Latin "ligare", to bind)
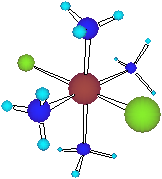
These transition
metal complexes are
commonly called coordination compounds.
commonly called coordination compounds.