†††††††††††††††††††††††††††††††††††††††† L1 / ††
8
CH1012

SN2
MECHANISM

1o†alkyl halides RCH2-X always
SN2
2o†alkyl halides
R(Rí)CH-X †††††† generally SN2
Experimentally
††† Rate (of
subs.)††† =††† k [RCH2X] [Nu:-]
Proposed
Mechanism
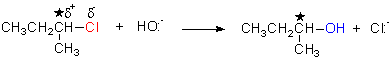
chiral centre

S
R
nucleophile†††††† leaving
††††††††††††††††††††††††††† group
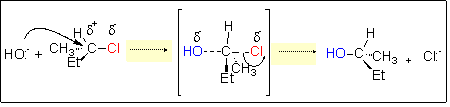
TS
plane
(Et = C2H5)