L1 /
2
CH1012

NMR: SPIN-SPIN
SPLITTING

B. Spin-Spin Splitting or Coupling.
• Protons (Hs) on adjacent
carbons will have different spin values ( +½
up ¯ -½ down) which give
different local magnetic fields that split apart the basic signal of the observed
signal.
• This is referred
to as coupling of spins
and the result is the splitting of a singlet signal into a multiplet (many peaks).
and the result is the splitting of a singlet signal into a multiplet (many peaks).
• Example: CHCl2CHF2
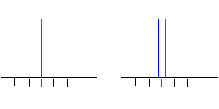
3.5 ppm
3.5 ppm
uncoupled
coupled