L1 /
18
CH1012

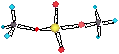
S-P ORBITAL
MIXING.h

•No s-p mixing
(E 2s << E2p)
If there
are some filled 2p orbitals eg. O,F,Ne
1. Es1s < Es2s
2. Es2s < Es2s*
3. Es2px < Ep2py, z (end
/ end overlap is greater)
s-p Mixing (E 2s ó E2p)
Only 1/2 filled 2p orbitals eg. B,C,N
reduced
repulsion in p AOs E is lowered
1. Es2s decreases due to mixing 2s+2px
2. Es2px raises due to mixing
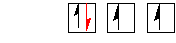
O 2s22p4
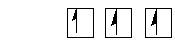
N 2s22p3