L1 /
16
CH1012

S AND P ORBITAL
DIAGRAMS

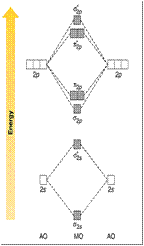
• Looking at the molecular orbital diagram shown
we have assumed that
the energies of the 2s and 2p
orbitals are far apart.
the energies of the 2s and 2p
orbitals are far apart.
• As a result the MOs that
form from these AOs have
the s2p bonding orbital lower
in energy than the p2porbitals.
form from these AOs have
the s2p bonding orbital lower
in energy than the p2porbitals.
• If, however, the energy of the
2s orbital is similar to the 2p
orbitals then the diagram
changes as mixing of orbitals
with similar symmetry occurs.
2s orbital is similar to the 2p
orbitals then the diagram
changes as mixing of orbitals
with similar symmetry occurs.