L1 /
10
CH1012

THE He2 MOLECULE

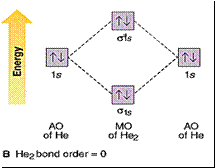
Bond order = 1/2 [no of e- in bonding MOs -
no of e- in antibonding MOs]
no of e- in antibonding MOs]
Bond order
> 0 means a stable molecule.
Bond order
= 0 means it doesn’t exist.
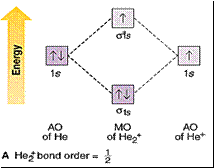
He2+
He2
doesn’t
exist
exists