L1 /
7
CH1012

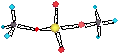

Ephoton = hn
= Eatom-highEstate - Eatom-lowEstate
Lowest E (closest) = Ground
state
Next highest E
= 1st
excited state
ATOMIC EMISSION SPECTRA
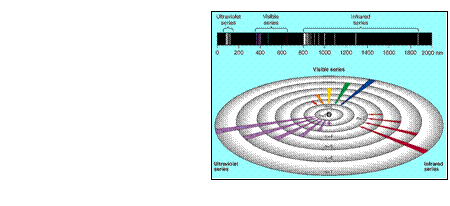
In the Bohr atom
each atomic state
corresponds to the
H electron (e-) being
- in a specific E level
- in an orbit a certain
distance away from
the nucleus.
corresponds to the
H electron (e-) being
- in a specific E level
- in an orbit a certain
distance away from
the nucleus.