L1 /
2
CH1012

ATOMIC STRUCTURE

Wave
particle / duality
ATOMIC STRUCTURE
Light has both wave and
particle properties.
Light travels as photons
eg. photoelectric effect
Light travels as photons
eg. photoelectric effect
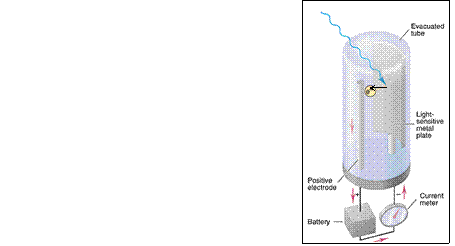
Light
of high enough frequency
(to exceed Ethreshold)
(to exceed Ethreshold)
photoelectron
emitted
E µ frequency
(particle theory)
not amplitude (wave theory)
not amplitude (wave theory)
and yet it diffracts
readily (wave).
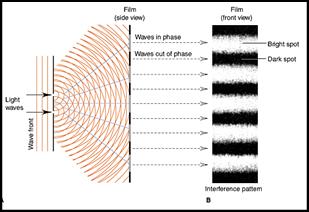
The electron also has both particle (cathode
rays) and wave properties (eg. diffraction).
rays) and wave properties (eg. diffraction).
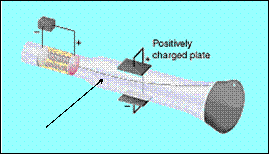
Cathode Ray Tube
Electron beam
Visible
light has Wave properties:
Wavelength l (nm) and frequency n (s-1 = Hz)
Wavelength l (nm) and frequency n (s-1 = Hz)
EM
radiation in vacuum has speed c (c = l n)
E = h n energy is proportional to n (Greek nu)
h = 6.63 x 10-34 J s Plank’s constant
c = 3.00 x 108 m/s Speeed of light
h = 6.63 x 10-34 J s Plank’s constant
c = 3.00 x 108 m/s Speeed of light